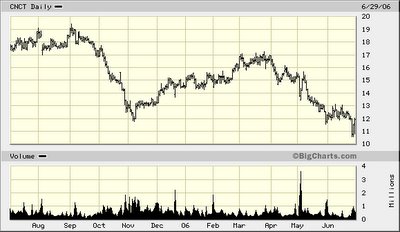
Itchy investors have pushed the sell button on the dermatology company, Connetics Corp. (CNCT-$11.65) driving the stock price 31% lower since March 2006, due to a rash of potentially injurious news releases:
- On March 28th, the SEC filed a civil lawsuit against a Connetics employee, Alexander Yaroshinsky, Vice President of Biostatistics and Clinical Operations, for alleged insider trading. According to a complaint filed in federal court, Mr. Yaroshinsky made at least $680,000 by trading Connetics stock soon after learning the Food and Drug Administration's (negative) preliminary views on the company's proposed acne drug, Velac Gel, which (because of tumorigenic concerns) was later deemed unsafe by the agency. [CNCT-$16.60]
- On Friday, April 8, 2006, Connetics announced that the SEC was widening its formal investigation into whether the company or any of its employees, officers or directors may have violated federal securities laws. [CNCT-$15.27]
- After the close of trading on May 3, the Company announced that after an investigation, it found that its reserve provisions for rebates—contractual discounts offered to government programs and private health plans when prescriptions are dispensed—were understated by about $8 million to $9 million as of Dec. 31, 2005. The estimated increased rebate reserve amount represents approximately 1.7% of cumulative total reported net sales for Connetics' four products.
By recording the additional rebate reserve to the balance sheet, aggregate historic net sales would be reduced by the amount of the reserve provision and net income and earnings per share would be reduced as well.
On a conference call, management also lowered revenue guidance due to increased competition. Total revenues are now expected to be $211 million to $217 million, compared with prior guidance of $221 million to $225 million, reflecting increased competition in the psoriasis market. Diluted EPS for 2006 is projected to be in the range of $0.44 to $0.50, including an estimated $6.8 million or $0.17 per diluted share in stock-based compensation expense. [CNCT- $13.76]
- On May 19, 2006, the Company announced that due to the delay in filing its Form 10-Q for the period ended March 31, 2006, the Company had received a letter from the The Nasdaq Stock Market indicating that the Company's Common Stock was subject to potential delisting pursuant to Nasdaq Marketplace Rules.
The Company, however, has requested a hearing before the Nasdaq Listing Qualifications Panel, thereby automatically deferring the de-listing of its common stock pending the Panel's review and determination. Until the Panel issues a determination and the expiration of any exception granted by the Panel, Connetics' Common Stock will continue to be traded on The Nasdaq National Market. [CNCT - $13.26]
- After the close of trading on Tuesday, May 30, 2006, Connetics received a Failure to Comply notice from Convertible Note holders. Specifically, management said that its debt holders communicated to the Company that its failure to file its first-quarter report for the period ending March 31, 2006, on time represented a breach of indentures governing the notes.
According to the Company, the notices were from debt holders who claim to hold more than 25 percent of each of the company's outstanding 2 percent and 2.25 percent convertible senior notes. In March 2005, the Company issued $200 million of 2.00% convertible senior notes in a private placement; and on May 28, 2003, Connetics issued $90 million of 2.25% convertible senior notes due May 30, 2008, in a private placement. Breaches could be interpreted as a potential defaults on its convertible debt obligations.
The Company has sixty days to remedy this breach (by filing its 1Q:06 10Q with the SEC). [CNCT -$12.60]
Yaroshinsky, 52, of Mountain View, Calif., and Zak were neighbors in Massachusetts in 1992, according to the lawsuit.
As a result of their improper trading, Yaroshinsky allegedly made more than $680,000, while Zak allegedly benefited by more than $900,000, the SEC said in its proposed amended lawsuit. [CNCT-$11.96]
Now that we have profiled the operating climate facing the Company, the question remains—Is now a good time to purchase shares of the Common Stock of Connetics?
First, the 10Q Detective believes that management (and the Board of Directors) are “worth their salt,” are motivated, and possess the experience and leadership necessary to remedy existing problems and to continue to build Connetics into a leading dermatology company.
Top executives are tenured, with years of pharmaceutical, wholesaler, and/or experience in working with clinical trials and the submission process to the FDA.
Additionally, the Board of Directors reads like a “Who’s Who” of Biotechnology & Government. For example, a Director since 1995, G. Kirk Raab, former CEO of biotech behemoth Genentech (DNA- $80.13) and the first chairman of the Biotechnology Industry Organization (BIO); Leon E. Panetta, a Director since 2000, served as President Clinton’s White House Chief of Staff; John C. Kane, Director since 1997, was the President and COO of drug wholesaler, Cardinal Health, Inc., from March 1993 until his retirement in December 2000; Denise M. Gilbert, Ph.D., a Director since 2003, has ties to Wall Street. From 1986 through 1993 Dr. Gilbert was a Managing Director and senior biotechnology analyst at Smith Barney Harris & Upham and Vice President and biotechnology analyst at Montgomery Securities; Carl B. Feldbaum, Director since 2005, served as President of BIO from its founding in 1993 until January 2005, and was former Chief of Staff to Senator Arlen Specter of Pennsylvania;
Management is motivated to see the Company through its sea of troubles. What better motivation than to know that all of the stock option grants awarded to executive management in FY 2005 are drowning—as they are all more than 50% out-of-the-money. Thomas G. Wiggins, CEO, was issued 135,000 option grants in 2005 at an exercise price of $23.35 per share; C. Gregory Vontz, President and Chief Operating Officer, was issued 90,000 option grants at an exercise price of $23.35; and, John L. Higgins, Executive Vice President-Corporate Development and Chief Financial Officer, was granted 81,000 shares.
We like Connetics because it is a company that knows its strength—the therapeutic dermatology category—and it has not tried to stray beyond its core competence. In our opinion, over time, Connetics will be able to drive more value to shareholders this way.
Connetics is a specialty pharmaceutical company that currently markets four products for the medical dermatology marketplace, Luxíq (betamethasone valerate) Foam, 0.12%, OLUX (clobetasol propionate) Foam, 0.05%, Soriatane (acitretin), and Evoclin (clindamycin) Foam, 1%.
Connetics’ proprietary foam delivery system used in Luxiq Foam, OLUX Foam and Evoclin Foam, has significant advantages over conventional therapies for dermatological diseases. The foam formulation liquefies when applied to the skin, and enables the active therapeutic agent to penetrate rapidly. When the foam is applied, it dries quickly and leaves minimal residue, and no stains or odor. The Company believes the cosmetic elegance of the foam improves patient compliance and satisfaction. In market research sponsored by Connetics, more than 80% of patients said they preferred the foam to other topical delivery vehicles.
.Luxíq Foam competes in the mid-potency topical steroid market while OLUX Foam competes in the high- and super-high potency topical steroid market. According to NDC Healthcare, or NDC, for the 12 months ended December 2005, the value of the total retail topical steroid market was $1 billion.
.OLUX Foam is a foam formulation of clobetasol propionate, one of the most widely prescribed super high-potency topical steroids. OLUX Foam has been proven to deliver rapid and effective results for scalp dermatoses, and for scalp and non-scalp psoriasis. Luxíq Foam is a foam formulation of betamethasone valerate, a mid-potency topical steroid prescribed for the treatment of mild-to -moderate steroid-responsive scalp dermatoses such as psoriasis, eczema and seborrheic dermatitis.
.Topical steroids are used to treat a range of dermatoses, for which approximately 30 million steroid prescriptions are written annually.
.While the topical steroid market is highly fragmented, Connetics believes OLUX Foam is the number one branded super-high potency topical steroid prescribed by U.S. physicians, and Luxíq Foam is the number one branded mid-potency topical steroid by retail sales and the third most commonly prescribed mid-potency topical steroid by U.S. dermatologists in 2005. Net product revenues for OLUX Foam and Luxiq Foam were $61.8 million (33.5% of total net revenue) and 24.1 million (13.1% of total revenue) in 2005, respectively.
Connetics acquired the exclusive U.S. rights to Soriatane from Hoffmann-La Roche, Inc., or Roche, in March 2004. Soriatane is an approved oral therapy for the treatment of severe psoriasis in adults. According to NDC, the value of the entire retail market for psoriasis was $875 million in 2005. Soriatane is currently the only oral retinoid indicated for psoriasis in the U.S. Net product revenues for Soriatane were $72.6 million (39.4% of total net revenue) in 2005.
Evoclin is approved for the treatment of acne vulgaris (the common form of acne seen in young adults, characterized by overactive oil glands that become plugged, red, and inflamed), and competes in the topical antibiotics market for the treatment of acne. Evoclin Foam is Connetics’ first commercial product that addresses the acne market.
According to the National Institute of Arthritis, Musculoskeletal and Skin Disorders, in the U.S. an estimated 17 million people are affected by acne annually, and an estimated 5.8 million people visited a physician for treatment during the 12 months ended September 2005. Industry sources indicate that the topical acne market is the largest segment of the U.S. dermatology market, generating approximately $1.3 billion in prescriptions in 2005, and that the active ingredient clindamycin is one of the most widely prescribed for acne in the U.S., with total revenues over $500 million in 2005.
Acne can be treated topically or systemically. Evoclin Foam competes primarily in the topical antibiotic acne market, representing approximately $599 million in U.S. prescriptions during the 12-month period ended December 2005. Connetics received FDA approval to market Evoclin Foam in October 2004 and began selling the product in December 2004. Net product revenues for Evoclin Foam were $24.7 million (13.4% of total net revenue) in 2005.
The Company has one New Drug Application (NDA) currently under review by the FDA, and two other product candidates for which management expects to file NDAs during 2006.
In November 2005, Connetics submitted an NDA for Desilux Foam, a low-potency topical steroid for the treatment of atopic dermatitis, formulated with 0.05% desonide in a proprietary emollient foam delivery vehicle, VersaFoam-EF. In January 2006, the FDA accepted the NDA for filing with a user fee date of September 21, 2006.
In September and November 2005, the Company completed two Phase III clinical trials designed to evaluate Primolux Foam, a super high-potency topical steroid (0.05% clobestasol propionate in a proprietary emollient foam delivery vehicle). Management plans to submit an NDA for Primolux Foam in the first quarter of 2006.
In July 2003, the Company submitted an NDA for Extina Foam, an investigational new drug formulation of 2% ketoconazole formulated using Connetics’ proprietary platform foam delivery vehicle for the treatment of seborrheic dermatitis. In November 2004, Connetics received a non-approvable letter from the FDA for Extina Foam based on its conclusion that Extina Foam did not demonstrate statistically significant superiority to placebo foam. Following continued discussions with the FDA, the Company initiated a Phase III trial of Extina Foam in September 2005, intended to demonstrate that Extina Foam is superior to placebo foam. Pending positive results from this Phase III trail, management anticipates submitting a Class 2 Resubmission for Extina Foam to the FDA by the end of 2006.
Connetics also owns worldwide rights to a number of unique topical delivery systems, including several distinctive aerosol foams. The Company has leveraged its broad range of drug delivery technologies by entering into royalty-bearing license agreements with several well-known pharmaceutical companies around the world.
· Liquipatch is a multi-polymer gel-matrix delivery system that applies to the skin like a normal gel and dries to form a very thin, invisible, water-resistant film. This film enables a controlled release of the active agent, which Connetics believes will provide a longer treatment period. Management anticipates developing one or more new products in the aerosol foam or gel matrix formulations, by incorporating leading dermatological agents in formulations that are tailored to treat specific diseases or different areas of the body.
In 2001, Connetics entered into a global licensing agreement with Novartis Consumer Health SA for the use of its Liquipatch drug-delivery system in topical anti-fungal applications. Novartis anticipates initial European launch of a product using the Liquipatch technology in 2006.
· Rogaine Foam. In 2002, the Company entered into a license agreement with Pfizer, Inc.) pursuant to which Connetics granted Pfizer exclusive global rights, excluding Japan, to its proprietary foam drug delivery technology for use with Pfizer’s Rogaine hair loss treatment. The FDA approved Rogaine Foam in January 2006. Pfizer has not yet announced its launch plans.
· OLUX Foam. In September 2004, Connetics entered into a license agreement granting Pierre Fabre Dermatologie exclusive commercial rights to OLUX for Europe, excluding Italy, where the product is licensed to Mipharm S.p.A. The license agreement with Pierre Fabre also grants marketing rights for certain countries in South America and Africa. Pierre Fabre will market the product under different trade names. Under the terms of the license agreement with Pierre Fabre, Connetics received an upfront license payment, and will receive milestone payments and royalties on product sales. Pierre Fabre anticipates an initial launch of OLUX in select European markets in 2006.
Dermatological disorders represent significant growth markets, since treatments are often under-treated or are less than efficacious. Granted, Connetics has its fair share of problems, but we believe for risk-tolerant investors, the Company is trading at an attractive valuation—16.2 multiple to forward December ’07 FY earnings estimates of $0.72 per share. This is only an 11% discount to the specialty pharmaceutical P/E multiple of 18 times, but forward earnings do not include a premium for the next generation foam products sitting in the Company’s pipeline.
Investment Risks. Delisting of Connetics from Nasdaq for failure to comply with timely filing of financial reports, convertible debt covenant issues (which could limit future financial flexibility), and/ or clinical and regulatory delays of products in pipeline could adversely affect the share-price of the Company’s Common Stock price.